-
Panagene¡¯s Innovative Synthesis
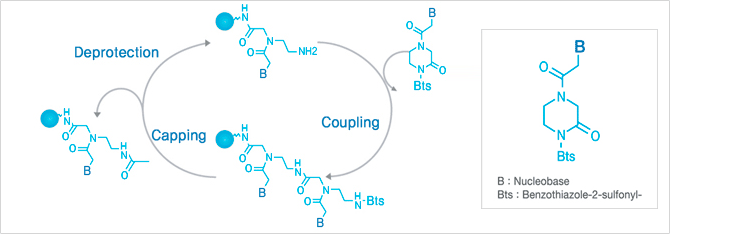
Panagene has developed its proprietary Bts PNA monomers (Bts ; benzothiazole-2-sulfonyl group) and proprietary oligomerization process. This unique structures enable PNA oligomerization to be more effective and convenient.
The PNA oligomerization using Bts PNA monomers is composed of repetitive cycles of deprotection, coupling and capping.
Panagene¡¯s PNA synthesis does not require anhydrous conditions or the pre-activation of monomers prior to the coupling reaction. It does not employ coupling reagents, and the excess monomers used in coupling reaction can be easily recovered and re-used (if needed). 
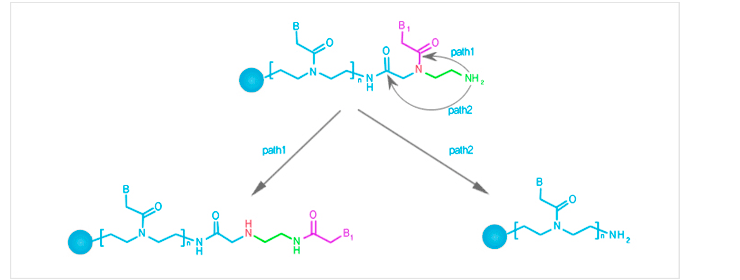
Under deprotection conditions used for the removal of Bts protecting group, transacylation of PNA is minimized.
Side reactions in PNA synthesis path 1 : trans-acylation of the nucleobaseacetyl moiety,
path 2 : N-terminal detachment of the monomer. -
Synthesis in General
The first synthetic strategy reported for PNA oligomer synthesis was Merrifield solid phase synthesis using t-Boc/Cbz protecting group strategy wherein the backbone amino group is protected with the t-Boc and the exocyclic amino groups of the nucleobases are protected with Cbz.
PNA monomers protected with t-Boc/Cbz are now available but are inconvenient to use because repeated treatment with TFA is required for t-Boc deprotection and the harsh HF or trifluoromethanesulfonic acid treatment required for cleavage from the resin and deprotection of Cbz group from exocyclic amine of nucleobases.
Alternative PNA monomers protected with Fmoc/Bhoc are also available wherein the backbone amino group protected with the Fmoc and the exocyclic amino groups of the nucleobases are protected with Bhoc. But Fmoc/Bhoc has also several drawbacks such as side reaction during the Fmoc deprotection process and instability of monomer in solution. The most critical side reaction is the migration of the nucleobase acetyl group from the secondary amino function to the free N-terminal amino function of PNA backbone under Fmoc deprotecting condition (trans-acylation). This trans-acylation reaction in every cycles during oligomer synthesis result in accumulation of side products which are hard to separate or characterize due to similar polarity and same molecular weight. Also the Fmoc protecting group is very unstable in the presence of trace amine. Thus, the selection of the solvent for the PNA monomers should be cautious. Generally, N-methylpyrrolidone of high quality is recommended.
Although the use of coupling agents such as HATU and HBTU have resulted in improved yields, there remains a need for new monomers and oligomerization process that increases yield, lowers synthetic cost, and is suitable for automatic and parallel synthesis. -
Comparison between Bts and Fmoc monomers
Panagene¡¯s proprietary method for the PNA synthesis using Bts monomers is superior to the other method that is publicly known
with Fmoc monomers.Criteria Panagene PNA
(Bts monomer)Other PNA
(Fmoc monomer)Cost of monomer production Low High Large scale synthesis of monomers Good - Solublity of monomers Good Moderate Stability of monomers in solution Good Poor Cost of oligomer synthesis Low High Solvent for oligomer synthesis DMF(Not necessary) NMP(Necessary) Coupiling reagent None HATU Pre-activation of monomers No Yes Transacylation during deprotection + ++++++ Reactivity of monomer with transacylated product little high Reuse of monomers Yes No